Power Morcellator Lawsuit
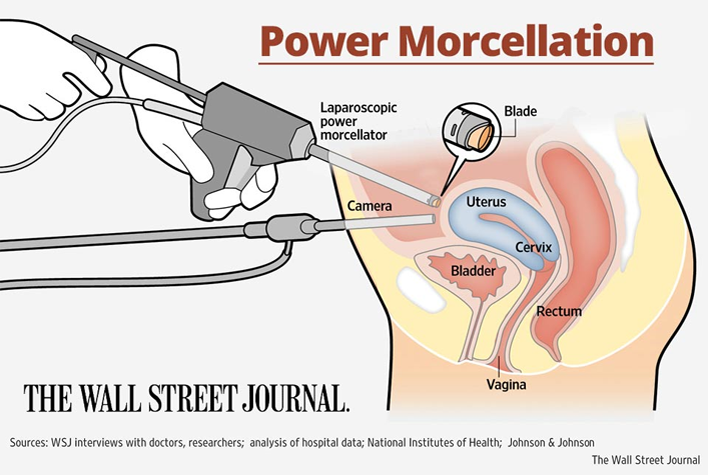
Lawsuits Johnson & Johnson subsidiary company Ethicon, Inc. has recently halted sales of their power Morcellators, as federal health regulators have issued warnings regarding the usage of the Power Morcellators. The Food & Drug Administration (FDA) report indicates the device can spread undetected cancer cells when undergoing surgery for hysterectomy or myomectomy for fibroids and have uterine sarcoma (cancer of the muscle & supporting tissues around the uterus).

FDA Warning::
Johnson & Johnson has stopped sales anddistribution of Power Morcellators as a result from the alerts issued by the FDA